New University of Florida project uses AI to improve treatments for childhood muscular dystrophy
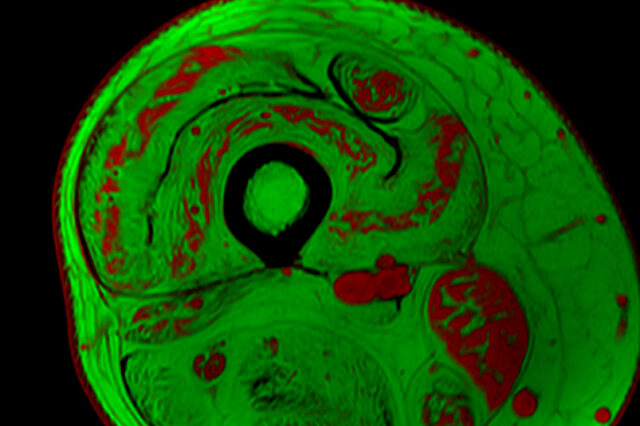
A project led by a researcher in the University of Florida's College of Public Health and Health Professions seeks to improve the effectiveness of clinical trials designed to treat childhood neuromuscular diseases.
Supported by a $480,000 award from UF President Ben Sasse's strategic funding initiative, the AI Applications to Pediatric Neuromedicine project combines a massive dataset of sophisticated muscle imaging data with UF’s powerful artificial intelligence computing.
“This project will lead to more effective treatments for devastating disorders that contribute to lifelong disabilities,” said Beth A. Virnig, Ph.D., M.P.H., the dean of the College of Public Health and Health Professions. “This project brings together a powerhouse of UF expertise in magnetic resonance imaging, neuromuscular disease, physiology, and AI to generate real-world improvements in health outcomes and enhance the quality of life for children with muscular dystrophy and their families.”
The new project is led by Krista Vandenborne, Ph.D., a distinguished professor and chair of the Department of Physical Therapy in the College of Public Health and Health Professions. Vandenborne pioneered the use of MRI biomarkers to capture highly accurate and noninvasive measures of muscle changes in children with Duchenne muscular dystrophy.
Neuromuscular diseases currently affect more than 1 million children and their families in the United States. These disorders can cause loss of reflexes, muscle weakness, and problems with balance and walking. In Duchenne muscular dystrophy, for example, muscles progressively weaken and lose the ability to regenerate after injury (so muscle tissue is eventually replaced with fat and collagen). Many children with Duchenne muscular dystrophy require a wheelchair by adolescence, and heart and respiratory systems are affected as the disease advances.
“In our decades of muscular dystrophy research, we have amassed a rich dataset of imaging and other biomarkers that represent millions of pieces of information characterizing the disease,” Vandenborne said. “Now, with the capabilities of AI, we can more quickly and accurately extract answers to treatment questions that will accelerate new therapies and bring us closer to a cure.”
Neuromuscular diseases are emerging targets for experimental gene therapies that may stop disease progression. However, assessing the drugs’ effectiveness in clinical trials can be difficult, particularly if the results are determined based on measures of participants’ functions (such as walking speed or ability to climb stairs). Among young children, especially, these measures can be hard to obtain if the child is not highly motivated to participate in functional tests or is just having a bad day. Muscle imaging, on the other hand, provides objective and accurate evidence of muscle changes.
“Drug developers have had little guidance or knowledge on how to best use imaging data in their studies of new therapies,” said Sarah Kim, Ph.D., an assistant professor of pharmaceutics in the UF College of Pharmacy. “With our application of AI to imaging data, we can provide faster, better analysis and instruction for pharmaceutical companies as they test new treatments.”
When paired with the pattern-recognition capabilities of AI, the comprehensiveness of UF’s muscular dystrophy dataset also offers the potential for providing diagnostic support (as well as predicting the course of an individual’s disease), said Glenn Walter, Ph.D., a professor and vice chair of the Department of Physiology and Aging in the UF College of Medicine.
“We have been following participants with muscular dystrophy for up to 10 to 15 years, and we have a good idea of the disease trajectory,” Walter said. “With machine learning and other AI applications, we believe that — in just one or two visits, with imaging and other tests — we will be able to predict the disease progression in a particular patient.”
Not only is the information valuable for clinical trials, Walter said, but it also is important for families. The knowledge could help families plan for wheelchair accommodations for their children and other changes to their homes, as well as inform their future treatment plans.
“At UF, we’re in the business of making life better. Enhancing the quality of life for children with neuromuscular diseases is such important work, and that is the aim of the AI Applications to Pediatric Neuromedicine project,” Sasse said. “By expanding investments in AI and imaging, advancements in neuromedicine will undoubtedly be made.”
About the author
