Scientists train AI to illuminate drugs’ impact on cellular targets

An ideal medicine for one person may prove ineffective or harmful for someone else, and predicting who could benefit from a given drug has been difficult. Now, an international team led by neuroscientist Kirill Martemyanov, Ph.D., based at The Herbert Wertheim UF Scripps Institute for Biomedical Innovation & Technology, is training artificial intelligence to assist.
Martemyanov’s group used a powerful molecular tracking technology to profile the action of more than 100 prominent cellular drug targets, including their more common genetic variations. The scientists then used that data to develop and train an AI-anchored platform. In a study that appears in the Oct. 31 issue of the journal Cell Reports, Martemyanov and colleagues report that their algorithm predicted with more than 80% accuracy how cell surface receptors would respond to drug-like molecules.
The data used to train the algorithm was gathered over a decade of experimentation. Their long-range goal is to refine the tool and use it to help power the design of true precision medications, said Martemyanov, who chairs the institute’s neuroscience department.
“We all think of ourselves as more or less normal, but we are not. We are all basically mutants. We have tremendous variability in our cell receptors,” Martemyanov said. “If doctors don’t know what exact genetic alteration you have, you just have this one-size-fits-all approach to prescribing, so you have to experiment to find what works for you.”
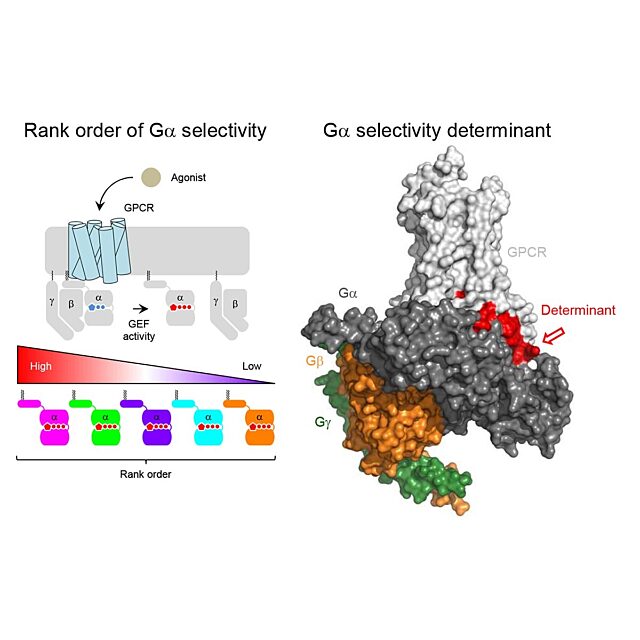
One-third of all drugs work by binding to cell-surface receptors called G protein-coupled receptors, or GPCRs. These are complexes that cross the cell membrane, with a “docking station” on the cell’s exterior and a branch that extends into the cell. When a drug pulls into its GPCR dock, the branch moves, triggering a G protein inside the cell and setting off a cascade of changes, like falling dominoes.
The result of activating or blocking this process might be anything from pain relief, quieting allergies or reducing blood pressure. Besides medications, other things like hormones, transmitters and even scents dock with GPCRs to direct biological activities.
Scientists have catalogued about 800 GPCRs in humans. About half are dedicated to senses, especially smell. About 250 more receive medicines or other known molecules. Martemyanov’s team had to invent a new protocol to observe and document them. They found many surprises. Some GPCRs worked as expected, but others didn’t, notably those for neurotransmitters called glutamate.
Martemyanov’s collaborators on the project included his postdoctoral researcher and later staff scientist, Ikuo Masuho, Ph.D., who now heads his own lab at Sanford Research in Sioux Falls, Iowa, as well as computational protein designer Bruno E. Correia, Ph.D., who is based at the Swiss Institute of Bioinformatics, in Lausanne, Switzerland, and was instrumental in creating the AI algorithm. Their collaboration grew from a lecture Correia gave at the Jupiter campus in Florida many years ago, Martemyanov said.
Martemyanov was struck by the fact that for an artificial intelligence algorithm to be useful, it must be trained with accurate data and clear rules. It was early days in GPCR research when they started, Martemyanov said, and they lacked that type of broad, sophisticated data on GPCR activity.
“If you’ve only looked at one leg of the elephant you may not have the right idea of how to describe it; you may not see that it’s actually an elephant,” he said.
Classifying GPCRs solely by their best-known activity was akin to seeing one leg of an elephant, he said. It was an oversimplification, too general to train AI, Martemyanov said.
To document the signaling in a comprehensive way, they turned to a useful technology called bioluminescence resonance energy transfer. It involved engineering a small bioluminescent tag into the cells’ proteins and documenting the change to the luminescence as the cell was exposed to molecules that activate GPCRs.
They gathered the data, attached ranks for binding preference and saw patterns emerge. The data resembled something like an EKG, with measurements for the activation rate, amplitude and selectivity. They added common genetic variants for the GPCRs humans carry, and documented significant differences in how these mutated receptors responded when activated.
When Correia’s group in Switzerland trained the algorithm to make predictions based on this more nuanced data, the researchers were excited by the results. They found it to be correct more than 80% of the time.
The scientists hope their results encourage drug developers to adopt a more sophisticated understanding of GPCRs, their G proteins and their activities in a way that ultimately benefits patients with safer medicines, created more quickly and at lower cost. Going forward, they intend to explore more deeply how genetic variation affects the way GPCR-acting drug-like compounds work.
“Our ultimate goal is to be able to predict how individual variants that people carry respond to drugs,” Martemyanov said, “allowing for the custom tailoring of prescriptions and paving the way for precision medicine.”
In addition to Martemyanov, Correia and Masuho, the co-authors of the study,“Rules and mechanisms governing G protein coupling selectivity of GPCRs,” were Ee Von Moo, Ph.D., Xiaona Li, Ph.D. and Hideko Wakasugi-Masuho, of The Wertheim UF Scripps Institute; Ryoji Kise, Ph.D., and Ryosuke Tany, Ph.D., of Sanford Research, and Pablo Gainza of the École Polytechnique Fédérale de Lausanne and Swiss Institute of Bioinformatics, Lausanne, Switzerland.
Support for the research was provided by the National Institutes of Health, grants DA036596 and MH105482; the Swiss National Science Foundation, the Swiss National Centre of Competence in Research (NCCR) in Chemical Biology, the NCCR in Molecular Systems Engineering, an EPFL-Fellows grant funded by an H2020 Marie Sklodowska-Curie, and startup funding from Sanford Research.